Clinical trials services and solutions
Quest Diagnostics supports clinical trials and academic studies with solutions for specimen collection, in vitro diagnostics (IVD) testing services, biopharma specialty lab services, and clinical trial recruitment.

Specimen collection
Mobile and in-center specimen collections give studies nationwide reach and support better participation. Our IVD lab services also allow us to provide deidentified remnant samples for clinical testing. We have a broad geographic reach via a network of more than 2,250 Patient Service Centers, with increasing retail presence including locations in Walmart® and Safeway™. Whether your participant population is spread across the country or you’re operating a virtual trial, the robust mobile phlebotomy network of ExamOne®, a Quest Diagnostics company, can help obtain the health data you need without sacrificing participant convenience.


In vitro diagnostic (IVD) evaluation testing solutions
Our dedicated Diagnostic Development team helps in vitro diagnostic companies bridge the gap between product concept and FDA and other regulatory approvals. We have an extensive testing menu, a broad offering of predicate devices, scientific and medical expertise, dedicated labs, clinical trial team members, and project management for all trials.
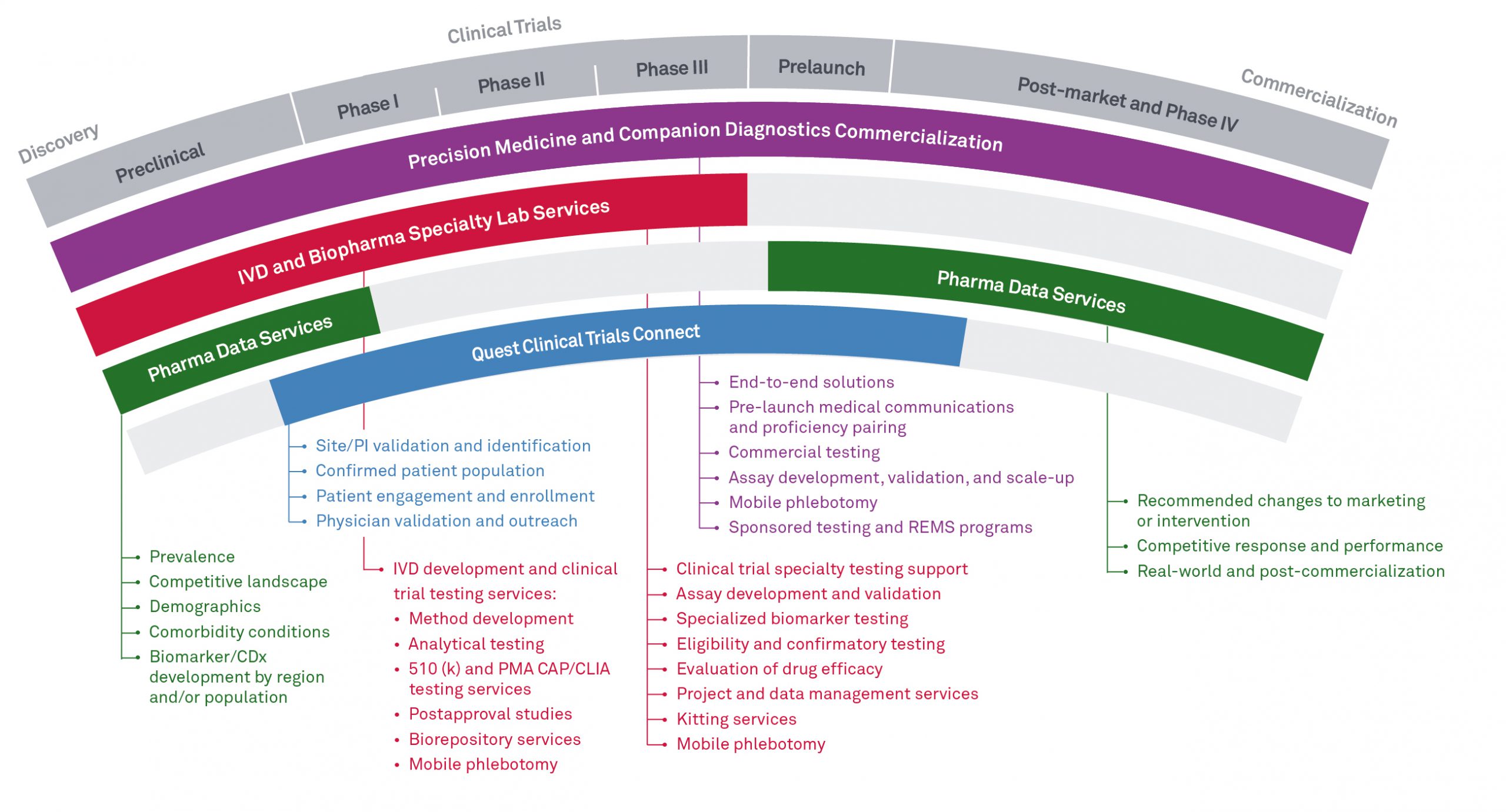
BioPharma Specialty Lab Services
Quest Biopharma Specialty Lab Services provides multi-site R&D and clinical testing services to support drug development and FDA clinical studies for IVD and BioPharma companies.

Clinical trial enrollment
Quest Clinical Trials Connect helps drive more effective and efficient clinical trials, connecting patients and physicians to clinical trial sponsors and clinical research organizations (CROs).
Site/investigator validation and identification
Using the inclusion/exclusion criteria set, we can validate matched potential patient volumes to sites and investigators and help identify additional physicians with concentrations of potential patients.

Patient recruitment
We connect patients with research study opportunities. We leverage identified patient relationships by reaching out to potential patients who have opted in to learn more about clinical trial opportunities and who match study criteria.

Physician outreach
We identify physicians and their patients who might qualify for clinical trials and inform them of the opportunity.
Quest Data Insights Platform™ powered by HealthVerity®
What you want to know, right away
Quest Diagnostics now offers a self-service search platform with immediate access to cleansed, ready-to-query, 60+ billion laboratory data results, going back 5+ years, making it easier to build patient and physician cohorts.
- Easily identify providers with patients who meet study criteria for a drug’s clinical trials
- Identify experienced principal investigators with patients whose profiles meet the criteria for your clinical trials

Put Quest Pharma Solutions to work for you