In vitro diagnostics (IVD) services
Comprehensive solutions that help bring new medical therapies through concepting and regulatory approval


Advancing IVD innovation
We’re committed to helping in vitro diagnostics (IVD) companies bridge the gap between product concept and regulatory approval. Our extensive testing menu, broad offering of predicate devices, scientific and medical expertise, medical technology and dedicated labs, clinical trials team members, and project management can facilitate a rapid development cycle across most IVD clinical trials.
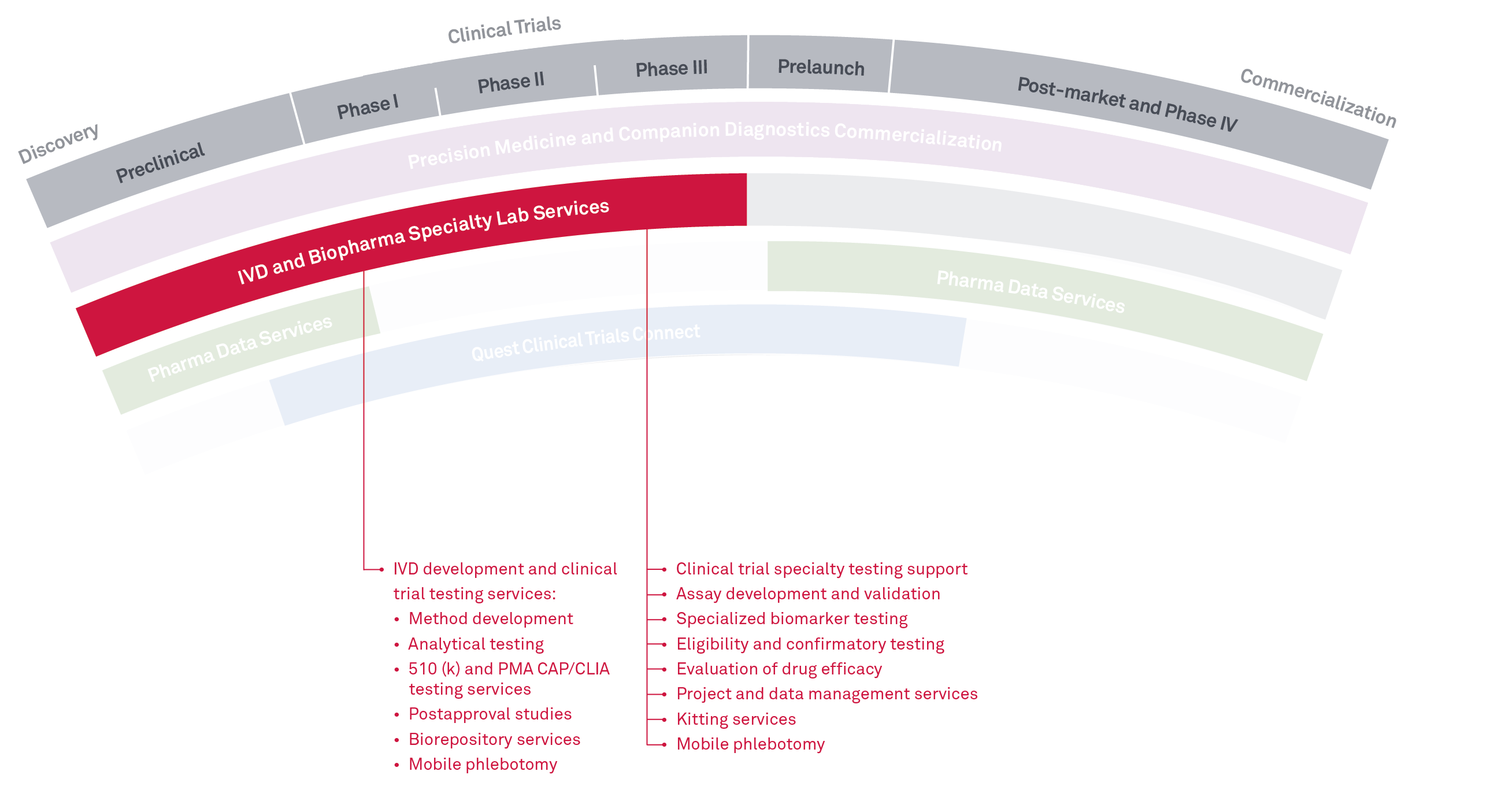
Combined with our Biopharma Specialty Lab Services, we provide multi-site R&D and clinical testing services to support drug development and FDA clinical studies for IVD and BioPharma companies. This includes eligibility, safety, and biomarker testing, as well as method development and validation supporting R&D and FDA submissions of assays and clinical instrumentation. We perform testing services across many disciplines with specialties in oncology, immunology, microbiology, infectious disease, women’s health, and molecular genetics.
We also offer boutique clinical trial services including custom reporting and data management services, customized project management, biorepository services, and expert consultation across all disciplines. We can offer thousands of tests through our laboratories and partnerships across the Quest network, and have a wide variety of platforms available.

We deliver on-time, on-scope, and on-budget clinical trial testing services that enhance your go-to-market timeline, including:
- Method development
- Analytical testing
- 510 (k) and PMA CAP/CLIA testing services
- Post approval studies
- Biorepository services
- Mobile phlebotomy
Learn more about how our Biopharma Specialty Lab Services helps speed your clinical trial to market.
Quest IVD services features include:
More than 650+ world-class, scientific leaders and teams of medical technologists to perform testing
High volumes of positive and negative controls and deidentified remnant samples for use in testing
Access to a broad array of predicate devices
Dedicated, open-floor and flexible lab facility with access to multiple labs for multi-site studies
Biorepository services for sample acquisition and storage pre-/post-study
Access-controlled laboratories for testing and clinical trial services
Experience performing over 170 studies for the world’s leading IVD companies
Interested in learning more on how our IVD and BioPharma Specialty Lab Services can work for you?