BioPharma Specialty Lab Services
Leading clinical trials diagnostics to facilitate clinical and commercial success

Supporting every step of
the clinical trial
Quest BioPharma Specialty Lab Services provides multi-site R&D and clinical testing services to support drug development and FDA clinical studies for IVD and BioPharma companies.
This includes eligibility, safety, and biomarker testing, as well as method development and validation supporting R&D and FDA submissions of assays and clinical instrumentation. We perform testing services across many disciplines with specialties in:
- Oncology
- Immunology
- Microbiology
- Infectious disease
- Women’s health
- Molecular genetics
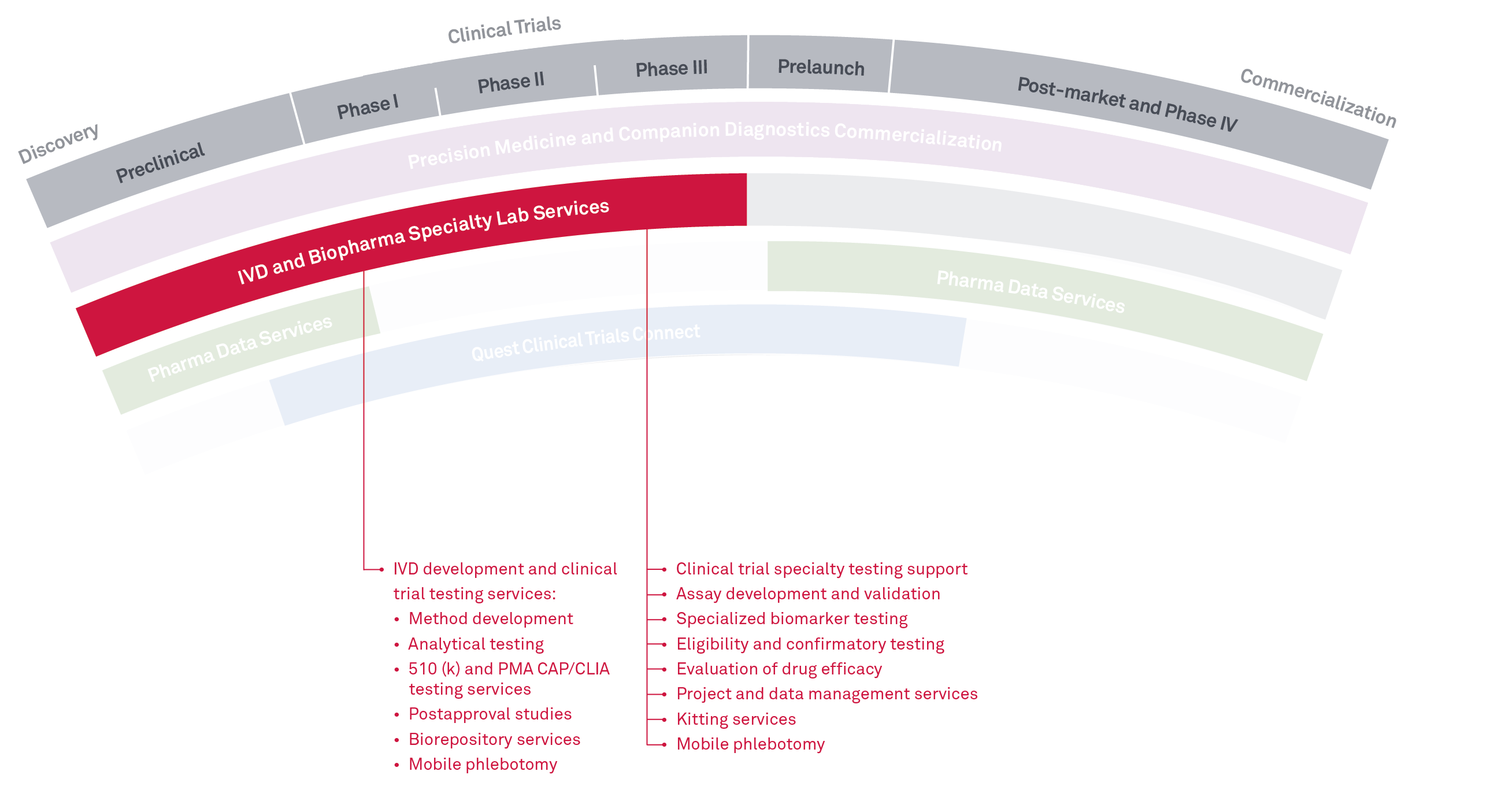
We also offer boutique clinical trial services including custom reporting and data management services, customized project management, biorepository services, and expert consultation across all disciplines. We can offer thousands of tests through our laboratories and partnerships across the Quest network and have a wide variety of platforms available.

Assay Development
- More than 3,000 assays performed across the organization for more than 20 years
- Access to one of the broadest test menus in the industry including novel and proprietary assays
- Assay target modulation
- Extensive menu of tissue-based and flow cytometric assays
- De Novo assay development
- Assay customization

Biomarker validation
- Selecting the right platform (NGS, individual analyte test, PCR, sequencing, IHC)
- Designing and optimizing custom assays
- Verifying, validating, and adding evidence for emerging biomarkers
- Providing access to medical, technical, and scientific staff

FDA filing
- Registration
- Pre-sub meeting planning and execution
- Documentation support
- Global approvals
- Pre-expertise (510(k); PMA; CE Mark)
Put Quest Pharma Solutions to work for you