The Power of Quest Lab Data
Helping to accelerate your product’s journey to the marketplace, safely

Typically, 70% of clinical decisions are made with lab data.1 This reliance on critical findings can help fuel tomorrow’s clinical and commercial strategy for drug development. Data-driven, predictive insights backed by lab data have the ability to dramatically change how pharmaceutical executives make strategic decisions.

Unlocking the power of lab data by linking to other key data
With more than 650 active EMR interfaces, Quest enables universal test ordering and high-availability results reporting. Many of our pharma and public health customers have invested in clinical data sets that are particularly relevant to their disease focus. Quest has built a series of collaborations with data providers that enable these clients to link those data sets with matched, de-identified data to help them understand the patient journey, patterns in disease progression, and acuity stratification.
For example, a therapy company may have a cardiovascular pipeline and investment in an events database containing stroke and heart attack data. Quest can leverage its own laboratory data to perform analyses to match relevant biomarkers, so that intervention opportunities and linkages between labs, medication history, claims, and outcomes can be explored. Quest has invested in multiple de-ID collaborators, so our clients can choose from 1 of 6 deidentified output formats, based on their previous investments.
The ‘Quest’ for an Effective Partner in Pharma and Biotech
How Quest Diagnostics can be an effective partner for pharma and biotech firms.
Quest lab data features include:
- A decade-plus of historic insights containing billions of data points reflective of national demographics
- Results from 4000+ tests, from the common to the complex
- Data from more than 313 million patients using 2,250+ Patient Service Centers across the US
- A flexible data pool size that can accommodate both common and rare diseases
- Historical data pulls and prospective feeds, with nearly real-time data, lagging less than a week
- HIPAA compliance
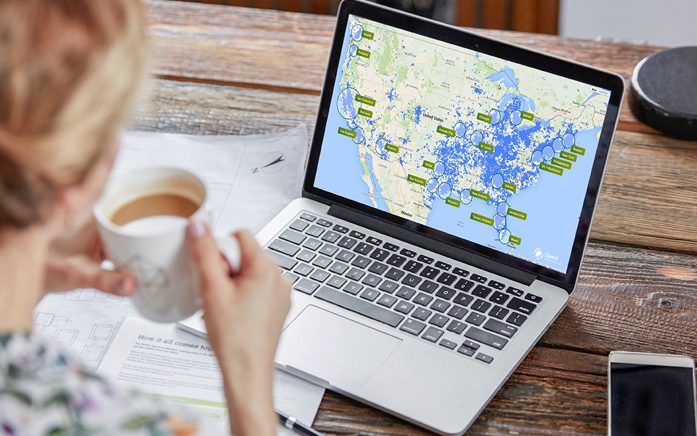
Fusing de-identified data and analytics with the right insights can provide a clear picture of a broad range of medical conditions and patient populations, driving efficiency and improving the quality of care for millions of Americans.
Comprehensive test menu
From routine testing to advanced genetic testing, our data covers nearly all therapeutic areas and can be leveraged across the drug development continuum. Quest Diagnostics data represent a diverse selection of therapeutic areas and medical conditions including:
- Allergy/asthma
- Arthritis
- Autoimmune diseases
- Bleeding and thrombosis
- Cancer, general
- Cancer, GI
- Cancer, leukemia and lymphoma
- Cancer, lung
- Cancer, skin
- Cancer, thyroid
- Cardiovascular
- Celiac disease
- Diabetes
- Endocrinology
- Gastroenterology
- General health
- Genetics
- Hepatitis B
- Hepatitis C
- HIV
- Infectious diseases
- Irritable bowel syndrome
- Neurology
- Pediatrics
- Prescription drug monitoring
- Rheumatoid arthritis
- Skin disease
- Toxicology
- Women’s health

Quest Diagnostics provides specialized data that help uncover unique insights via:
- Microbiology
- Genetic testing data
- Anatomic pathology data
- Chemistry tests
Quest Diagnostics data also feature important insights into key factors like physician specialty, diagnostic code at the time of the last order, and patient demographics.


Strategic alliances
- Alliances with Walmart® and Safeway® provide expanded access to lab testing in rural communities where people live and work
- Sophisticated, data providers that allow for linkage to de-identified data sets include:
- Datavant
- Hc1
- HealthVerity
- IQVIA
- Komodo Health
- Management Science Associates, Inc.
- Symphony Health
Facilitating the linkages between diagnostic laboratory insights and other data sets
Many of our pharmaceutical and public health clients have invested in clinical data sets that are particularly relevant to their disease focus. Quest has built a series of collaborations with data providers that create linkages between existing data sets and other matched, deidentified data to promote better understanding of the patient journey, patterns in disease progression, and acuity stratification.

National and third-party databases include:
- Census Data
- Centers for Disease Control and Prevention (CDC)
- US Department of Health and Human Services (HHS)
- Integrated third-party and client data for a robust, highly accurate data pool
HIPAA and protecting privacy
Quest Diagnostics is committed to protecting the privacy of identifiable health information, while advancing the science of healthcare and empowering better patient health. Throughout all stages of our research and reporting, maintaining patient privacy is paramount.
Our extensive data-collection process includes de-identifying data from multiple sources, which ensures that health information is anonymized. By protecting patient confidentiality while enabling our data scientists to analyze and interpret health trends, we can help our clients make more informed decisions on their journey toward effective disease interventions.
We follow the terms and conditions of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) explicitly. Any protected health information (PHI) data is de-identified, stored, and used for legitimate public health purposes in full accordance with the law.
Quest Diagnostics is a covered entity under HIPAA, which, with patient authorization, provides us with the ability to reach out to patients for clinical trial purposes as a care option. Quest also offers the use of our lab data to help identify the right clinically profiled patients who may be eligible for your clinical trials. We can also identify the physicians servicing these patients and reach out to them directly to educate them about your clinical trial.

Reference
1. Strengthening Clinical Laboratories. Centers for Disease Control and Prevention website. Updated November 15, 2018. Accessed June 21, 2021. https://www.cdc.gov/csels/dls/strengthening-clinical-labs.html
Put Quest Pharma Solutions to work for you